🗞️FDA clears the way for additional bivalent boosters for certain vulnerable individuals
🗿Semantically Similar Articles (by :title_embedding)
- 🗄️ 51.3 🔗 Apr03 FDA Approves New Antibiotic for Three Different Uses - FDA.gov (📰 Google News - US)
- 🗄️ 55.3 🔗 Mar30 FDA issues alert on heart pump linked to 49 deaths (📰 US general15)
- 🗄️ 55.4 🔗 Apr03 Vaccini: online 'Vax corner', dedicato all'informazione degli operatori sanitari (🧑🏻💻 webinfo@adnkronos.com (Web Info))
- 🗄️ 55.6 🔗 Apr02 Nuovi guai per von der Leyen: la procura europea indaga sulle sue trattative con Pfizer per i vaccini Covid (🧑🏻💻 repubblicawww@repubblica.it (Redazione Repubblica.it))
- 🗄️ 55.9 🔗 Apr02 Freight railroads must keep 2-person crews, according to new federal rule (📰 ABC News: US)
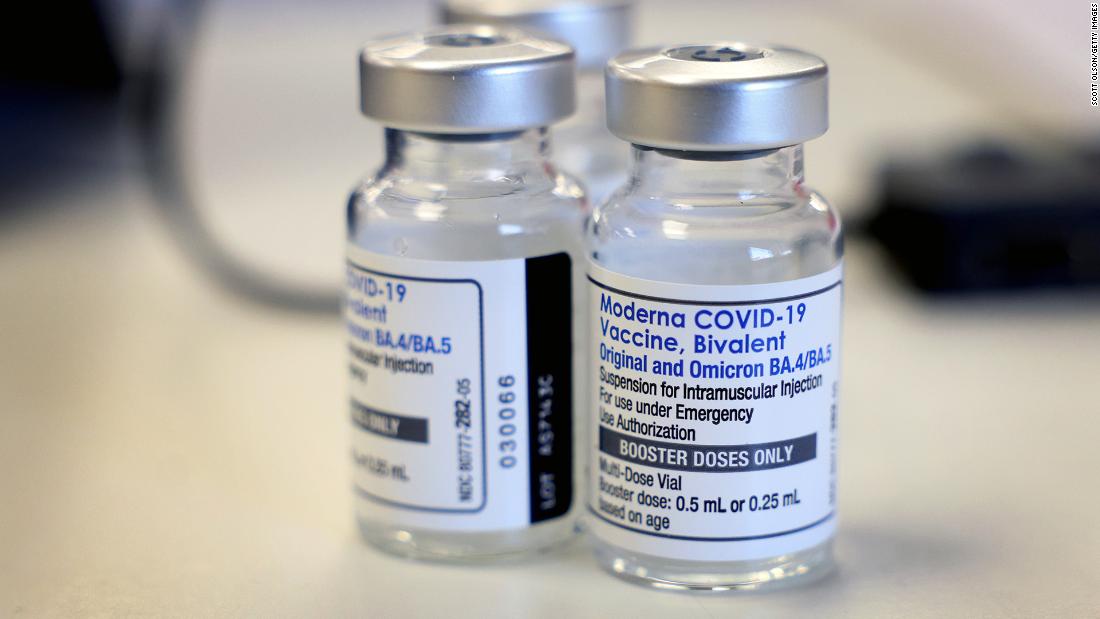
FDA clears the way for additional bivalent boosters for certain vulnerable individuals
2023-04-18
-
(from US general2)
The U.S. Food and Drug Administration amended the terms of its emergency use authorizations for the Pfizer and Moderna bivalent vaccines on Tuesday, allowing people ages 65 and older and certain people with weakened immunity to get additional doses before this fall's vaccination campaigns.
empty
[🧠] [v1/3] title_embedding_description: {:ricc_notes=>"[embed-v3] Fixed on 9oct24. Only seems incompatible at first glance with embed v1.", :llm_project_id=>"unavailable possibly not using Vertex", :llm_dimensions=>nil, :article_size=>539, :poly_field=>"title", :llm_embeddings_model_name=>"textembedding-gecko"}
[🧠] [v1/3] summary_embedding_description: {:ricc_notes=>"[embed-v3] Fixed on 9oct24. Only seems incompatible at first glance with embed v1.", :llm_project_id=>"unavailable possibly not using Vertex", :llm_dimensions=>nil, :article_size=>539, :poly_field=>"summary", :llm_embeddings_model_name=>"textembedding-gecko"}
[🧠] As per bug https://github.com/palladius/gemini-news-crawler/issues/4 we can state this article belongs to titile/summary version: v3 (very few articles updated on 9oct24)
🗿article.to_s
------------------------------ Title: FDA clears the way for additional bivalent boosters for certain vulnerable individuals Summary: The U.S. Food and Drug Administration amended the terms of its emergency use authorizations for the Pfizer and Moderna bivalent vaccines on Tuesday, allowing people ages 65 and older and certain people with weakened immunity to get additional doses before this fall's vaccination campaigns. [content] empty [/content] PublishedDate: 2023-04-18 Category: USA NewsPaper: US general2
"title"=>"FDA clears the way for additional bivalent boosters for certain vulnerable individuals",
"summary"=>"The U.S. Food and Drug Administration amended the terms of its emergency use authorizations for the Pfizer and Moderna bivalent vaccines on Tuesday, allowing people ages 65 and older and certain people with weakened immunity to get additional doses before this fall's vaccination campaigns.",
"content"=>"",
"author"=>nil,
"link"=>"https://www.cnn.com/2023/04/18/health/fda-bivalent-booster-additional-doses/index.html",
"published_date"=>Tue, 18 Apr 2023 15:09:15.000000000 UTC +00:00,
"image_url"=>"https://cdn.cnn.com/cnnnext/dam/assets/230418103631-covid-vaccine-booster-bivalent-super-169.jpg",
"feed_url"=>"https://www.cnn.com/2023/04/18/health/fda-bivalent-booster-additional-doses/index.html",
"language"=>nil,
"active"=>true,
"ricc_source"=>"feedjira::v1",
"created_at"=>Sun, 31 Mar 2024 20:37:37.038749000 UTC +00:00,
"updated_at"=>Mon, 21 Oct 2024 16:27:35.739279000 UTC +00:00,
"newspaper"=>"US general2",
"macro_region"=>"USA"}